What's Happening?
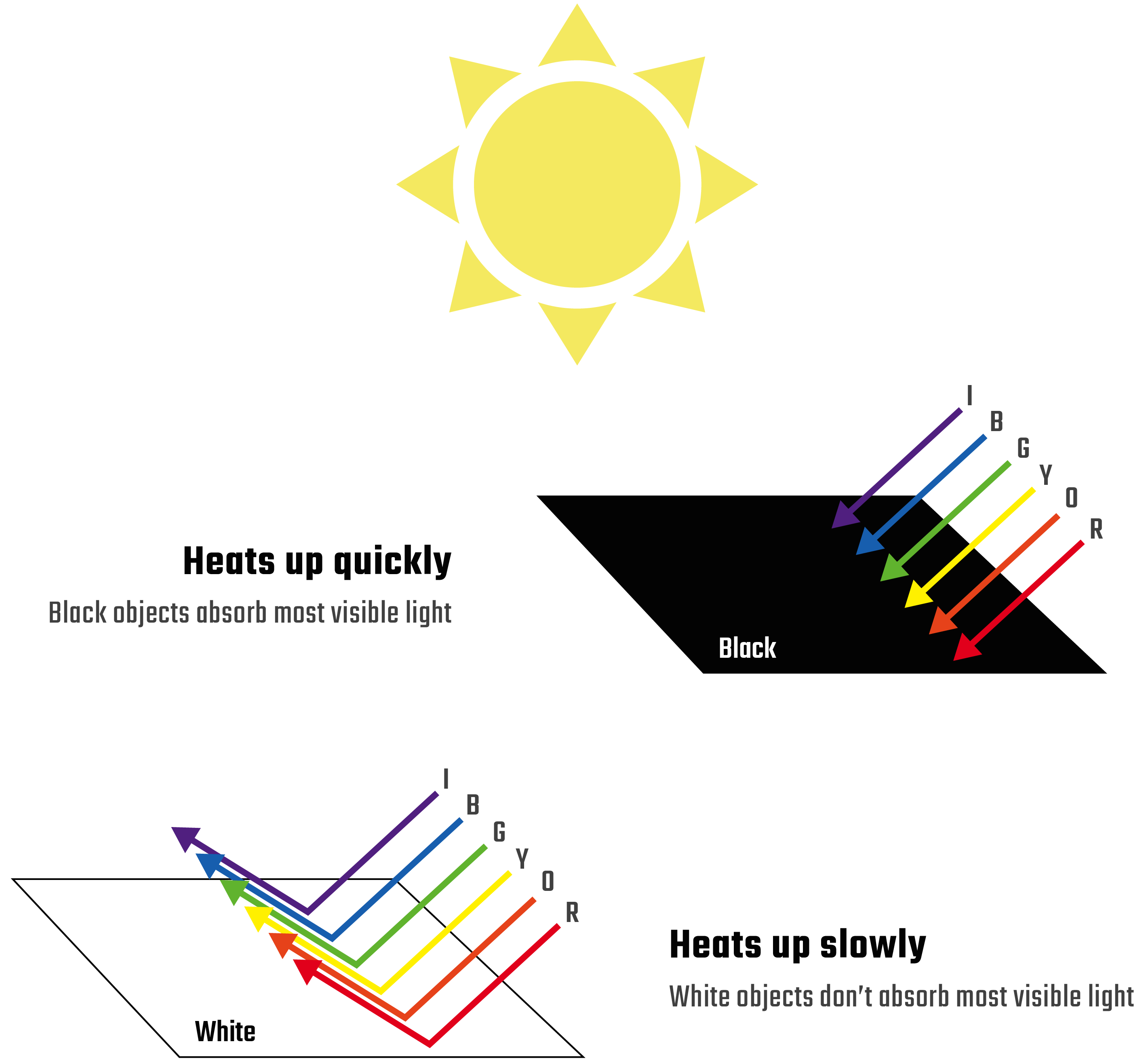
Sunlight is composed of many wavelengths of light, including visible wavelengths. These visible wavelengths are the colors that you can see! When sunlight hits a material, it’s absorbed as heat energy. The more sunlight absorbed, the warmer the material becomes. However, not all materials absorb the same types of light. Darker-colored materials absorb many wavelengths of visible light which makes them heat up fast. Lighter-colored materials don’t absorb as much light, so they heat up slower.
But what gives a material its color? The color is produced by the light that the material doesn’t absorb. For example, materials that don't absorb red light will look red to our eyes. So, the amount that a colored material heats up depends on the colors of visible light it absorbs.
Sunlight that reaches Earth has more red light in it than blue light. Since blue dye absorbs the more abundant red light, blue paper should heat up faster than red paper, which does not absorb that light. Does this match up with what you observed in your experiment?